Procedural Options & White Papers
Renú® Voice and Renú® Gel are provided in sterile ready-to-use 1.5cc syringes. No skin or allergy testing is required. Renú® Voice and Renú® Gel are designed to be easy to use and placed under direct observation allowing for exact control of volume delivered.
Placement and volume delivered should comply with suggested guidelines:
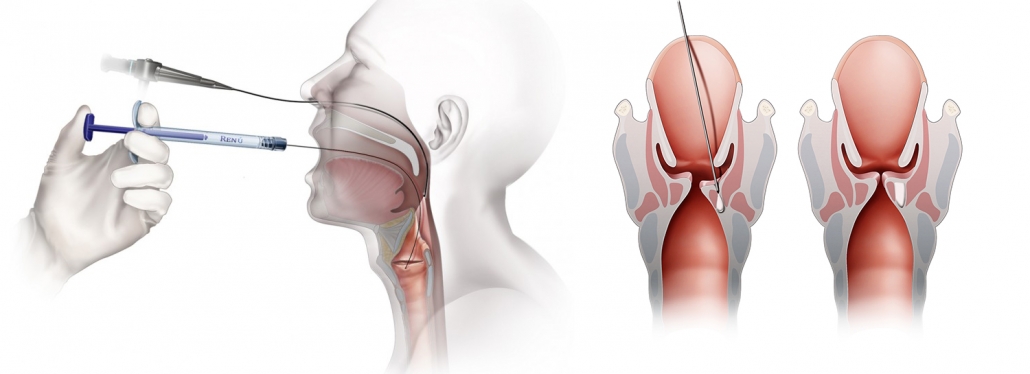
- Vocal Fold Injections should occur at the level of the vocal process
- Vocal Fold Injections should be at a depth of 5 -7 mm.
- Vocal Fold Injections should be under direct clinical observation; with an over correction of 10 – 15% is suggested.
Renú® Voice and Renú® Gel Injection Options
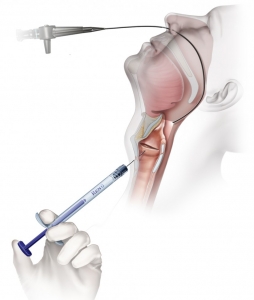
- Transoral (Office based)
- Percutaneous (Office based)
- Transoral (Operating Room)
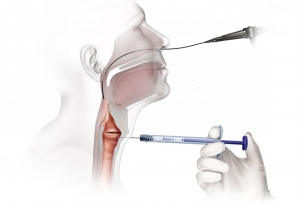
Percutaneous Vocal Fold Injection
Thyroid Cartilage Approach
Office-Based Transoral Procedure
- Topical anesthesia (dripped on larynx)
- Attach appropriate needle to Renú® Voice and/or Renú® Gel syringe
- Prime needle with Renú® Voice and/or Renú® Gel (dead volume equals ≈0.18cc)
- Special Note – record syringe starting and ending volume for exact volume delivered to patient.
Transoral Calcium Hydroxylapatite (CaHA) Injection
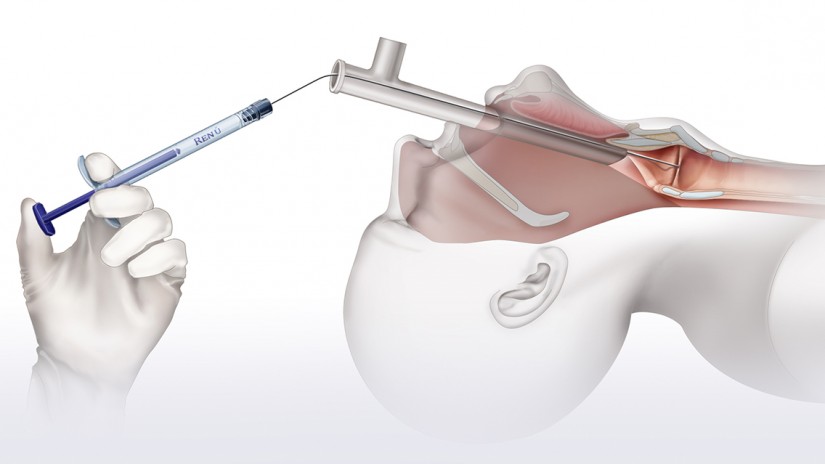
Operating Room (OR) Transoral Injection
- Visualization via direct laryngoscopy or micro-laryngoscopy
- Inject with approximately 10 – 15% over correction
Clinical Information
Peer Reviewed Journals
Rosen, C.A., J. Gartner- Schmidt,(et al.), “Vocal fold augmentation with calcium hydroxylapatite (CaHA). Otolaryngology Head and Neck Surgery (2007) 136, 198-204
Rosen, C.A., J. Gartner- Schmidt,(et al.), “Vocal fold augmentation with calcium hydroxylapatite (CaHA): Twelve month report. Presented on May 19, 2006 at the Annual Meeting of the American Bronco-Esophageal Association, Chicago, IL.
Rosen, Clark A. (et al.), “Vocal Fold Augmentation with Injectable Calcium Hydroxylapatite: Short-Term Results.” Journal of Voice, September 2004, Vol. 18, No. 3. p. 387-391.
Belafsky, Peter C. (et al.), “Vocal Fold Augmentation with Calcium Hydroxyapatite.” Otolaryngology – Head and Neck Surgery, October 2004, Vol. 131, No. 3, p. 351-354.
Chhetri, Dinesh K. (et al.), “Injection Laryngoplasty with Calcium Hydroxylapatite Gel Implant in an In-Vivo Canine Model.” Annals of Otology, Rhinology & Laryngology, April 2004, Vol. 113, No. 4, p. 259-264.
Hughes, Richard G. M., “Vocal Cord Medialization by Transcutaneous Injection of Calcium Hydroxylapatite.” Journal of Voice, December 2005, Vol. 19, No. 4, p. 674-678.
Kwon, T. K. (et al.), “Preliminary Results of a New Temporary Vocal Fold Injection Material.” Journal of Voice, December 2005, Vol. 19, No. 4, p. 668-673.
Product Questions
Where Can I Purchase Renú® Voice And Renú® Gel?
Contact Cytophil, Inc. by email (orders@cytophil.com) or toll-free call (+1 855-433-2765). Please include contact name, email address, and/or phone number with message.
Does Renú® Voice And Renú® Gel Harden After Injection?
The short answer is no. Since osteoblasts and osteoclasts do not exist above the periosteum, there are no catalytic cells to stimulate hardness.
Should I Expect Any Patient Side Effects?
Few side effects have been reported and those that are tend to center on those associated with injected implants. Tenderness and mild swelling at the injection site are commonly reported.
How Long Will Renú® Voice And Renú® Gel Last In My Patients?
Renú® Voice is comprised of two main components, each dissipating over different periods of time. The quickest to be absorbed is the carrier gel which takes a few weeks. Next the CaHA particles are broken down gradually into calcium and phosphate ions metabolized by the body’s natural process over a few months. Thus, Renú® Voice should provide patients long-lasting results for non-permanent restoration and augmentation of vocal folds. Typical Renú® Voice vocal fold implant can be expected to last from 9 to 18 months.
Renú® Gel is a hydrogel, that is quick to be absorbed by the body, which takes a few weeks. Thus, Renú® Gel should provide patients shorter-term results in comparison to Renú® Voice, for non-permanent restoration and augmentation of vocal folds. Typical Renú® Gel vocal fold implant can be expected to last from 10 to 14 weeks.
Does Renú® Voice And Renú® Gel Require Special Storage?
No, Renú® Voice and Renú® Gel may be stored at ambient room temperature between 59°F and 90°F (15°C and 32°C). Renú® Voice and Renú® Gel may be stored if packaging is intact and undamaged for up to two years.
REMINDER: Renú® Voice and Renú® Gel are manufactured as a single use product and once opened should not be stored or reused.
How Is Renú® Voice And Renú® Gel Packaged?
Renú® Voice and Renú® Gel come in a sterile foil pouch containing a single-use pre-filled syringe (packaged volume of 1.5cc), inside a protective outer box. Upon opening the foil pouch, it is common to find water droplets that are consistent with the normal sterilization process. Renú® Voice and Renú® Gel are manufactured as a single use product and any post procedure product remaining in the syringe should not be stored or re-sterilized for future use.